research design
This study was a trial that utilized a pretest-posttest design. This study protocol was approved by the Research Ethics Committee of National Taiwan University Hospital (No. 201812145RINA). The ClinicalTrials.gov number was NCT05281497. Written informed consent was obtained from all participants before starting the study. All methods were performed in accordance with relevant guidelines and regulations (Declaration of Helsinki). Initial trial registration took place on January 7, 2019.
Study population
In this study, we prospectively enrolled ESRD patients receiving HD treatment from the National Taiwan University Hospital (NTUH) Yunlin Branch HD Center and Mingde HD Clinic from July 2019 to December 2019. NTUH Yunlin Branch is a regional teaching hospital located in the suburbs of Taiwan. Central Taiwan. Ming-De HD Clinic is his 41-bed HD clinic for stable HD patients. Patients received standard bicarbonate HD three times weekly using a disposable dialysis machine with a high-efficiency or high-flux membrane, targeting a Kt/V of at least 1.2.18. Inclusion criteria for patients were being diagnosed with ESRD and receiving HD maintenance for more than 3 months and being 20 years or older. Those who agreed to participate in the study signed an informed consent form. Exclusion criteria were inability to use a smartphone, impaired walking ability or mental illness, or hospitalization in the past 3 months. Participants will be excluded if they change their dialysis method or undergo a kidney transplant during the study period due to incomplete intervention.
Data collection
All participants underwent a brief interview to document their demographic profile. Diagnoses of comorbid conditions are recorded by clinically relevant medical history or physical examination. Because HD patients have increased cardiovascular risk and decreased exercise tolerance, the Physical Activity Readiness Questionnaire (PAR-Q)19 It was conducted before the intervention. The PAR-Q provided a safe pre-screening of candidates for exercise testing and prescription. Laboratory data including hemogram and serum biochemistry were measured according to her ESRD patient care routine according to the guidelines of the Taiwan Society of Nephrology. All blood samples were collected before dialysis before the first HD session of the week.
Intervention description
The researchers included a nephrologist and his assistant from the School of Health Industry Management at the National Yunlin University of Science and Technology. It was also supervised by a physical therapist and a nutritionist. Each participant was provided with a wearable device (heart rate smart wristband, GSH405-B6, Golden Smart Home Technology Corporation) (Figure 1). This wristband has been approved by the National Communications Commission of Taiwan (NCC verification code: CCAB16LP1430T3). The device can detect steps (0-120,000 steps, 1 step split), calories, and sleep time.Wearable devices have been validated in previous studies17,20.

Heart Rate Smart Wristband, GSH405-B6, Golden Smart Home Technology Corporation.
Each participant downloaded an app (WowGoHealth app) to connect to a health management platform (GSH AI Health Platform) (Figure 2). Exercise-related data such as participants' steps, distance, calories burned, and heart rate were collected through wearable devices. All participants were taught how to take photos of their meals and record a food diary using a smartphone app. All information collected was uploaded to a health management platform. Only researchers had access to the data on the health management platform. The researchers analyzed an average of three to five photos each day and calculated the calories and nutrients in the foods, including starch, protein, and fat, as well as the percentage of fruits and vegetables. The researchers provided dietary suggestions to the participants.
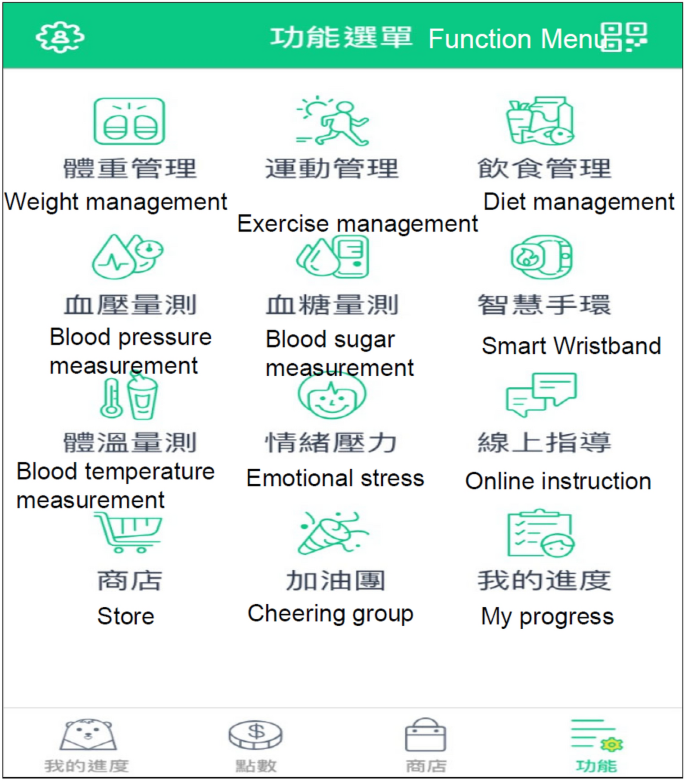
Health management infrastructure software user interface (WowGoHealth app).
Exercise included an 18-minute gymnastics program developed by the Health Promotion Bureau of the Ministry of Health and Welfare (Figure 3). The videos include stretching, aerobic training, and strength training, which can be performed in a seated or standing position. Participants were asked to perform calisthenics at least three times a week. Following a prospective cohort study, he set the number of steps per day at 7,500. The study found that mortality rates gradually decreased and plateaued at about 7,500 steps per day.twenty one.

(Quoted from official website: https://youtube.com/user/hpagov).
18-minute exercise video created by Health Promotion Bureau, Ministry of Health and Welfare Chinese version: https://youtu.be/_w50TfdCmKU; Hokkien version: https://youtu.be/-Otr4BlYm2E
LINE is a mobile app operated by LINE Corporation. All users can communicate with text, images, video, and audio at any time. LINE is the mainstream text messaging app in Taiwan. We created a LINE group to motivate participants, especially if they achieved their daily step goal. We implemented a telephone consultation service for health information via LINE. All interventions lasted 24 weeks.
Results measurement equipment
All outcome measures were collected at baseline and at 4, 8, 12, 16, and 24 weeks after study initiation.
Main achievements
The Sit to Stand 10 (STS-10) test measured the time it takes to complete 10 sit to stand cycles. Participants were instructed to begin and end the test in a seated position in a standard chair placed facing a wall. The patient rose from a sitting position and sat back down as quickly as possible with his arms crossed in front of his chest. The time taken to perform 10 repetitions was recorded.twenty two. Sit-to-stand-60 (STS-60) measures the number of sit-to-stand cycle repetitions achieved in 60 seconds.twenty three. STS-10 and STS-60 were suitable for measuring lower extremity muscle strength and had high test-retest reliability in ESRD patients.twenty three.
The 6-minute walk test (6MWT) was used as an index of exercise ability. 6MWT was performed in accordance with the American Thoracic Society statement.twenty four. The participant was instructed to walk as fast as he could down a flat 30-meter track for 6 minutes. They were allowed to stop and rest during the test, but were instructed to start walking as soon as they were able to do so.recorded the distance walkedtwenty three.
Hand grip strength (HGS) is an easy test to perform and correlated well with lean body mass in ESRD patients.twenty five.This was an independent outcome predictor in male she-ESRD patients26. The strength of each hand was measured using a hand grip dynamometer (CAMRY Digital Hand Dynamometer EH101, Figure 4). The device can automatically capture the electronic hand's grip. The Jamal dynamometer, which is widely recognized as a grip strength measurement tool, provided excellent reliability and validity.27. The participant performed the STS-10, STS-60, 6MWT, and HGS before his third HD session of the week.

Grip strength of each hand was measured using a CAMRY digital hand dynamometer EH101.
secondary results
HRQoL was measured by the Kidney Disease Quality of Life Survey (KDQOL-36™).28. KDQOL-36™ is a short form that includes the 12-item Short Form Questionnaire (SF-12) as a general core, as well as Kidney Disease Burden, Kidney Disease Symptoms/Problems, and Impact of Kidney Disease Scale from KDQOL. was. -SF™ v1.3. Items 1-12 were SF-12. Items 13–16 were the burden of kidney disease. Items 17-28 were symptoms/problems. On the other hand, items 29 to 36 were the effects of kidney disease. The higher the score, the better the HRQoL.Cronbach's alpha was estimated to be greater than 0.828.
Subjective global assessment (SGA) was a reliable tool for assessing nutritional status and detecting protein energy wasting (PEW) in dialysis patients29. Her SGA of 7 points was scored based on the patient's history of weight change over the past 6 months, dietary intake, and presence of gastrointestinal symptoms (anorexia, nausea, vomiting, diarrhea). Physical examination was performed for decreased subcutaneous fat mass and muscle wasting. Scores 6-7 indicate normal nutritional status, scores 3-5 indicate mild to moderate her PEW, and scores 1-2 indicate severe her PEW.30.
statistical analysis
Statistical analyzes were performed using IBM SPSS Statistics for Windows, version 22.0.0 (IBM Corporation), and two-sided analysis. P Values < 0.05 are considered significant. We examined the distribution of all outcome data. Distributional characteristics of the data were expressed as mean ± standard deviation for normally distributed continuous variables or median (interquartile range) for skewed distributed continuous variables. Results were compared using repeated measures ANOVA and F-test with Greenhouse-Geisser adjustment (F-GG) was used to correct for degrees of freedom. F-distribution31. For categorical variables involving percentages (%), the chi-square test or Fisher's exact test was used. Pearson or Spearman correlations (normally or non-normally distributed, respectively) were used to analyze the association between monthly step count and physical function, HRQoL, SGA, and laboratory data.
Ethics approval and consent to participate
This study was approved by the Research Ethics Committee of National Taiwan University Hospital (No. 201812145RINA), and informed consent was obtained from all participants. The ClinicalTrials.gov number was NCT05281497. His first trial registration took place on January 7, 2019.