In a recent study published in the journal nature communications, Researchers identified the influence of genetic and modifiable risk factors (MRFs) on brain networks vulnerable to aging, schizophrenia and Alzheimer's disease in around 40,000 UK Biobank participants.
 Research: The influence of genetic and modifiable risk factors on brain regions vulnerable to aging and disease. Image credit: Kittyfly / Shutterstock
Research: The influence of genetic and modifiable risk factors on brain regions vulnerable to aging and disease. Image credit: Kittyfly / Shutterstock
background
Developing strategies to modify risk factors could reduce the incidence of dementia and pave the way to healthy aging. This approach tests for a variety of factors, including cerebrovascular problems such as high blood pressure, diabetes, and obesity, protective measures such as exercise, and lifestyle choices such as alcohol intake. A comprehensive assessment of MRF, including lifestyle and environmental pollution, revealed that it may affect 40% of dementia patients worldwide. Despite the fixed nature of genetic factors associated with diseases such as Alzheimer's and Parkinson's, brain imaging reveals that certain regions are particularly vulnerable to aging and neurodegenerative diseases. I did. Further research is essential to unravel the complex relationship between genetic predisposition and modifiable lifestyle factors on brain health and the progression of neurodegenerative conditions.
About research
In the study, which utilized the UK Biobank imaging cohort, researchers included data from 39,676 participants who underwent structural T1-weighted brain scans. These scans were processed to map gray matter, focusing on identifying a network of brain regions labeled 'last in first out' (LIFO) that had previously been identified as particularly susceptible to the effects of aging. I guessed it. This network was characterized primarily by higher-order brain regions and was analyzed for their unique contributions to brain structure that set them apart from other regions.
This study complied with ethical guidelines, and UK Biobank obtained the necessary approvals and participant consent. Researchers looked at 161 MRFs across 15 categories, including those identified by the Lancet commission as being associated with dementia risk, excluding traumatic brain injury. This comprehensive selection aims to understand the impact of these MRFs on LIFO networks without reducing data complexity.
Statistical analysis began with genome-wide association studies (GWAS) to investigate genetic influences, followed by assessing the association of each MRF with the LIFO network. This study aimed to pinpoint the specific effects of these MRFs by adjusting for confounding factors such as age and gender. Further analysis included a combined model of all important MRFs to comprehensively evaluate their unique contributions.
Post hoc analyzes further investigated genetic factors, including assessing causal relationships within genetic clusters and performing enrichment analysis of gene function. Additionally, mediation analysis investigated the relationship between Alzheimer's disease-associated microtubule-associated protein tau (MAPT) gene variants and the LIFO network. This study also investigated the genetic overlap between MRF and LIFO phenotypes, providing insight into potential common genetic pathways.
research result
In this study, the LIFO brain network, which is known to be susceptible to aging, showed a significant quadratic relationship with age, with accelerated gray matter volume decline in higher-order areas related to cognitive functions such as executive action, working memory, and attention. It became clear that there was.
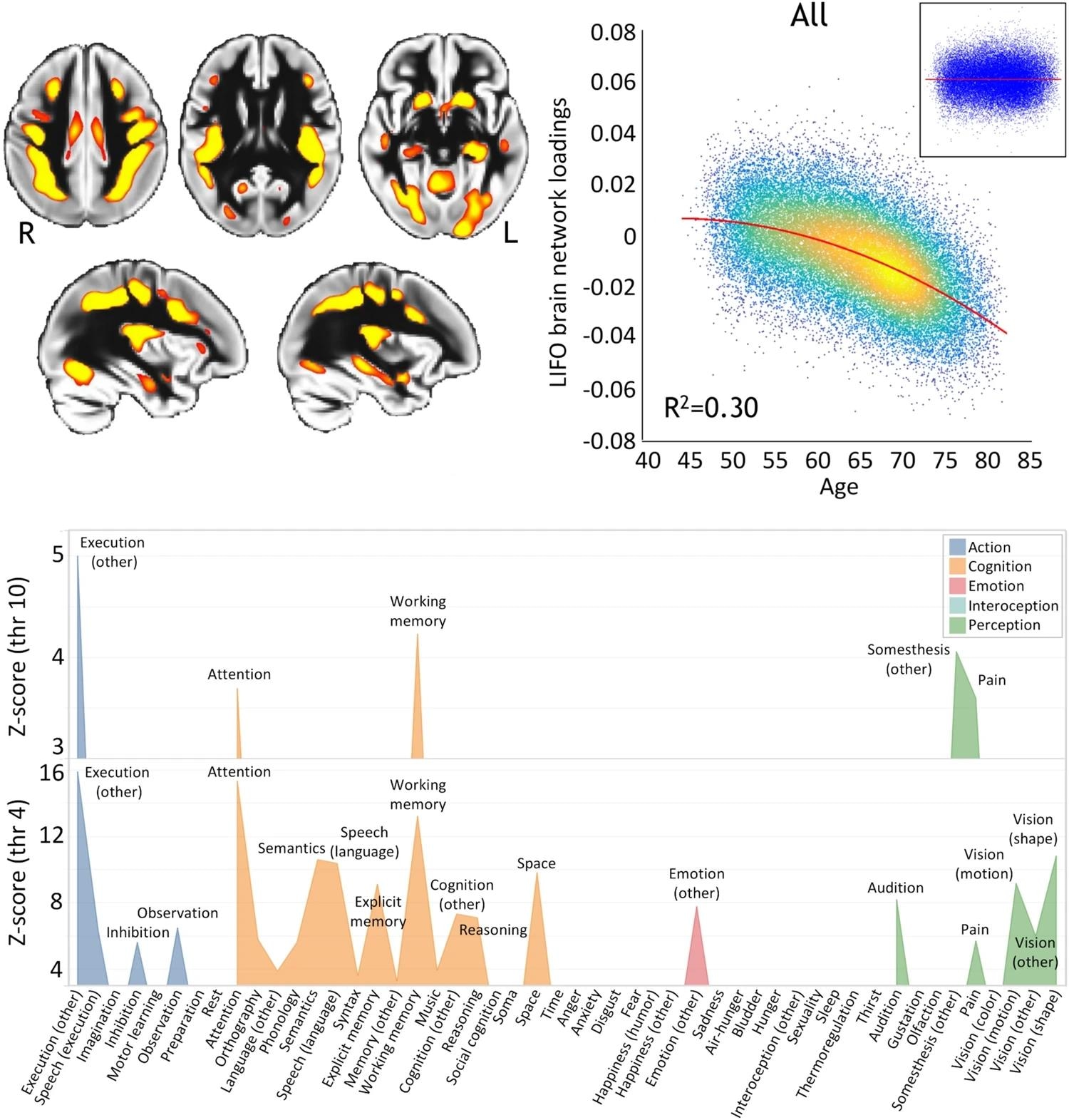 Top left, spatial map of the LIFO network used to extract loads from all participants scanned from the UK Biobank (red and yellow, thresholded at Z>4 for visualization). (n = 39,676). Top right, these LIFO loadings (in arbitrary units) show a strong quadratic correlation with age in the UK Biobank cohort. That is, in these specific regions, gray matter volume decreases quadratically with age (R2= 0.30, P < 2.23 × 10−308; Inset: residual scatterplot). Finally, the vulnerable network appears to primarily encompass regions related to execution, working memory, and attention (using the BrainMap taxonomy and thresholding at both Z = 4 and Z = 10 (using LIFO brain network)
Top left, spatial map of the LIFO network used to extract loads from all participants scanned from the UK Biobank (red and yellow, thresholded at Z>4 for visualization). (n = 39,676). Top right, these LIFO loadings (in arbitrary units) show a strong quadratic correlation with age in the UK Biobank cohort. That is, in these specific regions, gray matter volume decreases quadratically with age (R2= 0.30, P < 2.23 × 10−308; Inset: residual scatterplot). Finally, the vulnerable network appears to primarily encompass regions related to execution, working memory, and attention (using the BrainMap taxonomy and thresholding at both Z = 4 and Z = 10 (using LIFO brain network)
This study identified genome-wide associations between the LIFO network and seven genetic clusters, and the associations were replicated across all clusters. These genetic influences include potassium 2-pore domain channel subfamily K member 2 (KCNK2), which is involved in neuroprotection and inflammation control, and known solute carrier family 39 member 8/zinc iron regulatory protein 8 (SLC39A8/ZIP8). Contains clusters near genes such as . It is known for its wide range of associations with health markers and diseases. Other notable findings include close variants of runt-related transcription factor 2 (RUNX2), which is associated with neurogenesis and Alzheimer's disease, and NUAK family kinase 1 (NUAK1), which is associated with schizophrenia and depressive disorders. Contains mutants. The MAPT region, which has been implicated in several neurodegenerative diseases, also showed significant associations.
Two genetic clusters on the X chromosome, specifically in pseudoautosomal regions, were also identified. These clusters are associated with the XG blood group antigen and show associations with a variety of health outcomes, including nitrogen dioxide air pollution, highlighting the impact of the environment on brain health.
This study utilized a two-stage analysis to further explore the MRF and identify its impact on the LIFO network. Initial findings identified significant associations across 12 categories of MRF, with pollution, diabetes and alcohol consumption emerging as notable risk factors affecting his LIFO network. This comprehensive model, which takes into account confounding factors such as age and gender, highlights the multidimensional nature of brain health, combining genetic predisposition with environmental and lifestyle factors.
Although the heritability of the LIFO network was confirmed, genetic coheritability with Alzheimer's disease or schizophrenia did not show statistical significance. This finding suggests a complex interplay of factors that contribute to brain network vulnerability.
conclusion
In summary, in this study, researchers discovered important genetic and lifestyle factors that influence a brain network prone to premature aging, known as the LIFO network. They identified seven gene clusters, including novel ones on sex chromosomes, and highlighted diabetes, air pollution and alcohol as key modifiable risks. These findings reveal the complex interplay between genetics and environment on brain health and highlight the vulnerability of the LIFO network to aging and diseases such as Alzheimer's disease and schizophrenia. This study also opens new avenues for research into the genetic influence of XG blood type on brain aging.
Reference magazines:
- Manuello, J., Min, J., McCarthy, P. The influence of genetic and modifiable risk factors on other brain regions vulnerable to aging and disease. Nat Commun (2024), DOI-10.1038/s41467-024-46344-2, https://www.nature.com/articles/s41467-024-46344-2